ON THE HISTORY OF SALT EXTRACTION
For thousands of years salt has been a very important material: it was not just used for seasoning, but was needed much more as a preservative. Before ice boxes, fridges and finally freezers were invented, all foods from meat and fish to vegetables and even fruits had to be salted if they were to serve as long lasting provisions. Therefore, the use of salt can be traced as far back as Neolithic times. It was well known in the early civilisations of Mesopotamia and Egypt and in antiquity was already produced on a proto-industrial scale.
Apparently, the people of what is now Hallstatt in Austria were the first to manufacture salt in large amounts. The production of salt, which still continues today, is indicated by the name "Hallstatt", because the german syllable "Hall-" goes back to the greek word salt. In the Hallstatt mine people already chiselled big chunks of rock salt from the walls in the 14th century BC. Between 800 and 400 BC, in the first Celtic era, production virtually went through the roof. Whole families laboured in the wide mining spaces underground. The men, dressed in finely woven, coloured woollen capes, broke large, heart shaped pieces of salt from the walls. The people became rich in the salt trade: archaeologists found such an amount of leftovers from their wealth in the Hallstatt region, they termed the first flourishing period of Celtic culture "Hallstatt-period".
At the same time production hit a first peak in the most important salt producing region of France, the Seille valley in eastern Lorraine. There, salt was manufactured from the saliferous spring water in the area. Workers filled the salty water, called "brine", in big ceramic pans and heated them until the bulk of water had evaporated. Then they placed the concentrated brine on a "briquetage", a ceramic grid over a fire, supported by small pillars. There the brine sizzled until only a cake of pure salt was left.
The Romans were the first to use the third technique of salt production on a large scale: on the Meditarranean coast they gathered seawater in large shallow basins. Due to the intense sun and the wind, over time the water evaporated and in the end workers scraped up pure dried sea salt. These three traditional ways of salt production have already been described by the roman scholar Pliny the elder in his "Natural History".
The beginnings of many famous salt works which flourished for centuries lie in the early Middle Ages: Wielicka in Poland for instance, Lüneburg in Northern Germany, and a number of salines in Worcestershire in England. At that time salt refining became more efficient through the utilisation of large pans made of lead instead of ceramics, but still consumed a lot of energy. The consequences can be witnessed today in the environs of Lüneburg: the famous Lüneburg Heath only came into being because the local forests were ruthlessly exploited for the fires of the salt works. The most influential innovation in medieval salt production was the invention of leaching. Presumably it was used for the first time in Hallein in Austria's Salzkammergut: in the mine a space carved out of the salt rock was filled with fresh water which absorbed the salt from the surrounding walls. Thus an artificial brine was created which later would be boiled on a conventional briquetage. This new process opened up rock with low salt concentrations for exploitation, too.
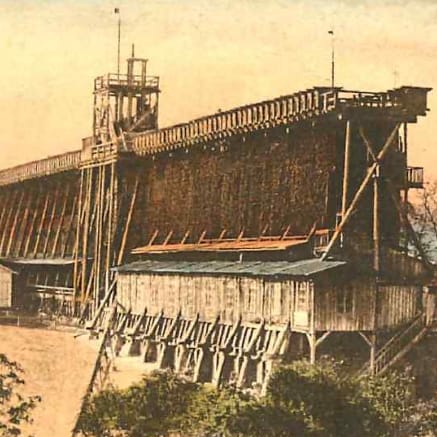
Since the middle of the 16th century, so-called "graduation houses" or "graduation lanes" came into being. These constructions, which presumably originated in Lombardy, can still be seen in many old salt producing towns. They were basically long thorny hedges of considerable height, which served to increase the salinity of the brine: If saliferous water was run over a blackthorn or juniper hedge, a certain amount of the water would cling to the twigs and thorns of the plants. Afterwards, less time and less firewood was needed to boil the brine. Graduation lanes quickly spread and some were extended almost to the length of a mile. A second, unexpected advantage of the installations emerged in the 19th century, when spa guests where advised to promenade along the graduation lanes and breathe the saliferous air.
Despite such efforts to reduce the necessary amount of firewood, in the middle of the 16th century a wood shortage began to be experienced. Increasingly, salt works had to try out the much detested, stinking coal as a combustible. So in fact it was the production of salt which initiated the rapid expansion of coal mining. Prussia for example, holding a profitable monopoly for the salt trade as many countries did, reorganized the entire mining sector to further increase the profits. Salt works in Great Britain though were quickest to adapt to the new fuel, and the country became the biggest manufacturer and also consumer of salt.
From the middle of the 18th century on, this process was further accelerated by the industrialisation in the UK, because salt now was increasingly sought for as a commodity for chemical industries. Particularly it was needed to produce soda, a bleaching agent in the booming cotton business. Originally, soda had been made from plants, but corresponding to an invention of the French chemist Nicolas Leblanc, it was synthesized now on a large industrial scale from sulphuric acid, coal, calcium carbonate and salt. The process reduced the cost of cotton products drastically, but was extremely hazardous to health and environment. Since it was rather expensive, too, it was replaced by a new procedure by the middle of the 19th century. Developed by Ernest Solvay from Belgium, this was based on natriumchloride, carbondioxide and ammonium. Because soda also served as a basic material in the manufacturing of glass and soap, Solvay's company grew to a potent chemical enterprise which still is in business worldwide today. Also the British company ICI earned its once leading position by producing soda.
Since the end of the 19th century, chemical industries have an increasing demand for potassium salts. Because potassium is a major nutrient for plants, potassium salts are particularly needed to synthesize fertilizers, but also for soap-, textile- and paper-production. The world's largest resources of pure potassium salt are to be found in a long stretch from east to west across Germany. The salt mines there can be recognized from far away by their whitish-grey mountain-like spoil heaps, which still pose a threat to the environment, since rains wash out the remaining salt.
In the 20th century salt has become a cheap everyday product, because new deposits have been opened up and production has been thoroughly economized. The techniques though are basically still the same as in centuries before. A striking example is "solution mining": in this highly efficient technique to extract rock salt, the medieval leaching process has been optimized by drilling holes into the rock and installing two pipes in each of them, one for injecting fresh water, which dissolves the salt from the rock, the other to extract the saturated brine. Later the brine is boiled as it traditionally was, only now in a more energy efficient, highly automated process.